2024. April 08.
In the journal Nature Communications, Gergely Nándor Nagy, a researcher of the BME, and his co-authors published a paper on significant results in understanding memory formation.
He is a colleague of Department of Applied Biotechnology and Food Science at the last year 150 years old Faculty of Chemical Technology and Biotechnology (BME), and collaborated with researchers at the University of Copenhagen and the University of Michigan during his postdoctoral research at Oxford University, the results of which have just been published in the prestigious Nature Communications open access journal [1].
The authors of the paper examined a crucial protein molecule present in the brain area responsible for memory formation. Essential new insights into the structure and interactions of this protein have been gained, providing the opportunity to identify the role of this molecule in neuronal development of mice. The results are of great importance for understanding how memory develops, as they can provide guidance for further research.
|
Proper brain development and adaptability are driven by a complex communication network of neural tissues, for which signals are provided by interactions between macromolecules on the cell surface. One such cell membrane-bound signaling protein is Semaphorin-5A (Sema5A). The growth of nerve cells is controlled by the interactions between the Sema5A protein and other various cell surface glycan polysaccharides (heparan sulfate and chondroitin sulfate). In the research, the authors of the paper uncovered the molecular background of these interactions. The binding site for Sema5A glycan was determined, then analyses in structure, solution and living cells demonstrated the protein and glycan molecule groups determining the interaction, and following that, genetically modified mouse model tests identified the crucial role of Sema5A-glycan interactions in the development of the dentate gyrus brain region. |
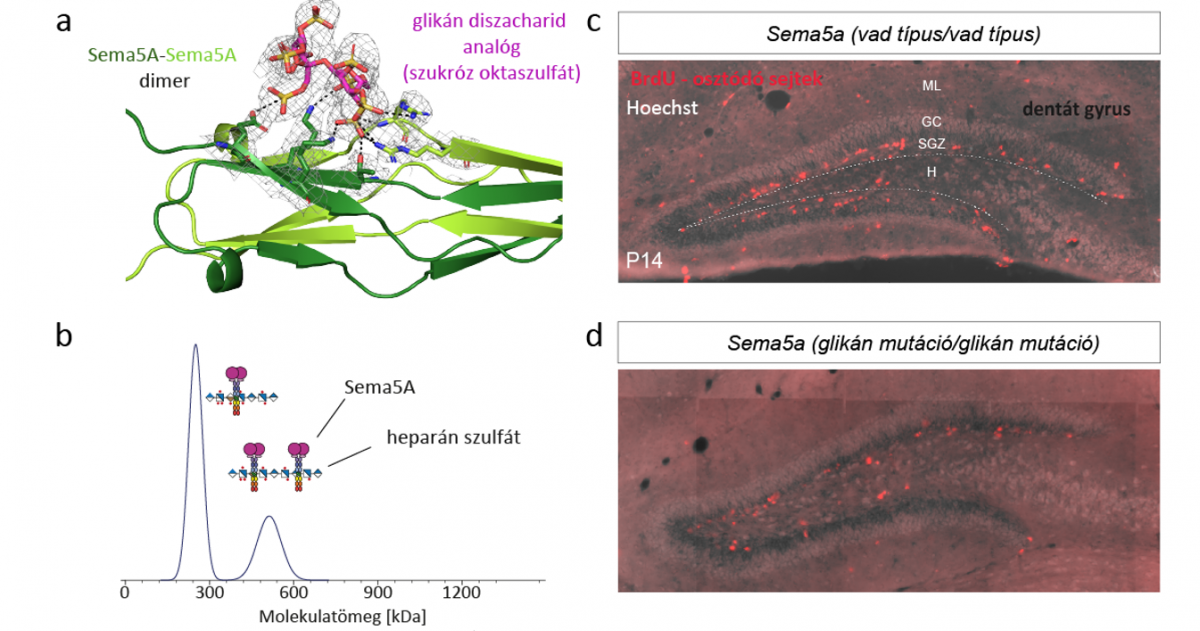
a) The Sema5A protein’s Complex crystal structure is a glycan disaccharide with analogue molecule. b) In solution, Sema5A forms oligomers with heparan sulfate polysaccharides. c-d) Due to the Sema5A mutation designed on the basis of structure, the original location of dividing neurons in dentate gyrus brain region of mice (c (bright pink, dot-like pattern) changed (d).
Gergely Nándor Nagy was interviewed about the research by bme.hu:
How many years of research were needed to achieve the results published recently?
The research took four and a half years from the first experiments to the final publication. More time was needed due to a number of circumstances arising from the nature of the research. During the international cooperation with a number of participants, new relationships were formed between the research groups. The results were built on each other and a full analysis of the previous examinations was necessary for planning further experiments.
How did you manage to collaborate continuously with the 11 other authors of the paper?
I am extremely grateful for the guidance and support of E. Yvonne Jones, my postdoctoral group leader. As the paper progressed, an excellent collaboration formed between the researchers involved. In addition to my own experimental results, I have been corresponding author with the three research team leaders, E. Yvonne Jones, Roman Giger, and Rebecca L. Miller, in recognition of my role in bringing the research together. I remain in active contact with many of the authors of the paper. I collaborate with my postdoctoral group leader on a number of research directions, and we are also co-supervisors of a PhD student at Oxford. My current research at the BME is carried out in collaboration with co-authors Rebecca L. Miller and Henrik Clausen from the University of Copenhagen.
Do the results mean that we are closer to understanding how human episodic memory works, and thus to being able to influence how it works?
Our paper is an important milestone in knowledge at the molecular level and can provide an explanation for some genetic neurological disorders. We hope that the new knowledge can be a starting point for research into memory formation; however, that requires numerous additional molecular and human neuroscience and cognitive studies.
|
Nature Communications is a multidisciplinary journal covering many scientific fields, which is part of the Nature Publishing Group portfolio, and publishes original communications. According to its own statistics, the publisher published 43 papers by Hungarian authors between December 2022 and November 2023 [2]. Citations: [1] Gergely N Nagy , Xiao-Feng Zhao , Richard Karlsson , Karen Wang , Ramona Duman , Karl Harlos Kamel El Omari, Armin Wagner, Henrik Clausen, Rebecca L Miller, Roman J Giger, E Yvonne Jones Structure and function of Semaphorin-5A glycosaminoglycan interactions Nature Communications (2024) DOI: 10.1038/s41467-024-46725-7 [2] https://www.nature.com/nature-index/country-outputs/articles/all/Hungary |
KJ-Rector’s Office Department of Communications